Danish Paper shows the incidence of vaccine adverse reactions varied with production batches
Public health authorities have failed in their duty of care
A new Danish peer reviewed research paper “Batch-dependent safety of the BNT16b2 mRNA Covid-19 vaccine”, published in European Journal of Clinical Investigation found from a study of 701 million administrated doses of the BNT162b2 mRNA vaccine, manufactured by Pfizer-BioNTech, in the European Union was linked with 971,021 reports of suspected adverse effects.
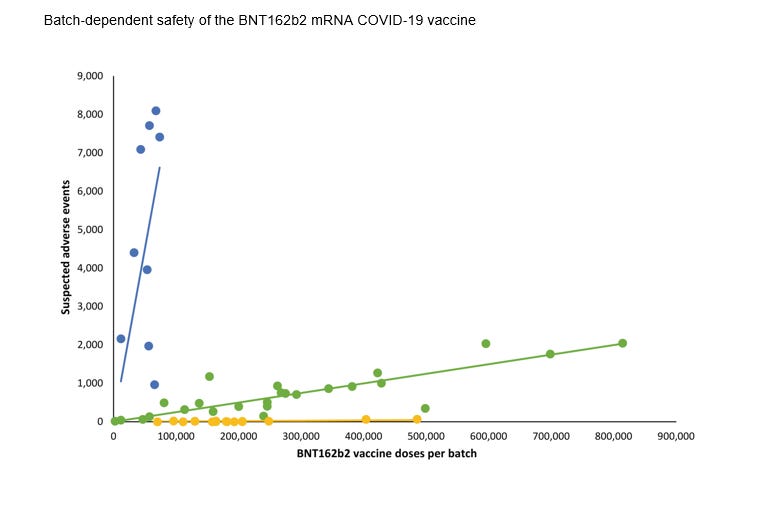
The study found out that the propensity of adverse effects varied according to batch.
From the paper: Numbers of suspected adverse events (SAEs) after BNT612b2 mRNA vaccination in Denmark (27 December 2020–11 January 2022) according to the number of doses per vaccine batch. Each dot represents a single vaccine batch. Trendlines are linear regression lines. Blue: R2 = 0.78, β = 0.0898 (95% confidence interval [CI] 0.0514–0.1281), green: R2 = 0.89, β = 0.0025 (95% CI 0.0021–0.0029), yellow: R2 = 0.68, β = 0.000087 (95% CI 0.000056–0.000118). Vaccine batches representing the blue, green and yellow trendlines comprised 4.22%, 63.69% and 32.09% of all vaccine doses, respectively, with 70.78%, 27.49% and 47.15% (blue trendline), 28.84%, 71.50% and 51.99% (green trendline), and 0.38%, 1.01%, and 0.86% (yellow trendline) of all SAEs, serious SAEs, and SAE‐related deaths, respectively.
The study evaluating data in Demark between 27th December 2020-11th January 2022, on 5.8 million people found 71% of suspected adverse reactions occurred in only 4.2% of the vaccine batches. The rates of suspected adverse reaction reports varied between batches. In one batch, there was 1 suspected adverse effects report per 20 doses of vaccine administrated.
There are a number of factors that could influence the propensity of adverse reactions from the vaccine. These include the vaccine manufacturing processes, storage, transportation, clinical handling and control. And administration techniques.
Another possibility was that the formula varied in the Pfizer manufacturing process from batch to batch. No public health authority or pharmaceutical registration body had ever followed up on this issue.
Why did this study have to be undertaken by independent researchers and not by Pfizer and the pharmaceutical regulatory agencies in their post market safety reviews?
Public health authorities around the world have failed to protect the safety of their citizens in failing to pick this fault out. In addition, Pfizer has not spoken out and provided any explanation for this.
The full paper can be accessed here.
Subscribe Below:





What the paper shows is that deliberate contamination of vaccine can happen, probably at source. This is a trial run which shows that deliberate contamination is a viable option. Now we know that contamination can occur, the next question is what contamination do you want to give. That's why Dr David Martin said that the vaccine is a bio terrorist weapon. Will you accept a gift of free vaccine in the future? Based on this paper, NO. China is smart. It refused free gift of mRNA vaccine from US.
Full paper link leads to a site no longer working