Developing new pharmaceutical products
Some notes on conceptualization to comercialization
Until 2020, new pharmaceutical products took a decade to create. Here are some notes on the traditional route of creating new pharmaceuticals from natural inspiration.
Pharmaceutical companies are taking more interest in the potential for the discovery of new compounds from plants. Cosmoceutical and nutraceutical companies are looking for more herbs and other natural products to meet the growing demand for supplementary foods and herbal preventative medicine. The food, cosmetic, herbal and pharmaceutical industries are merging, bringing many new products, which cannot be clearly defined. Within this environment, there are many new companies that are basing their product range and existence around particular technologies and natural active pharmaceutical type ingredients [159].
Today, the pharmaceutical industry can be divided into four areas;
1. Prescription drugs,
2. Over the counter drugs (OTC),
3. Health and food supplements, and
4. Traditional medicines and herbs.
Prescription medicines comprise both patented and generic drugs, where dispensing is restricted to doctors, pharmacists and hospitals. OTC, health and food supplements and traditional medicines may be sold by non-professional outlets and the public.
The nutraceutical sector comprises of herbal, health and dietary supplements which are usually regulated as non-poison over the counter (OTC) or as traditional herbal medicines. These supplements usually take the form of pills, powder, capsules, teas, or beverages. They contain enzymes, vitamins, amino acids and other natural herb extracts. Registration requires proof of efficacy, quality and safety, and production under GMP conditions. Prescription products are stringently tested before registration and undergo random surveillance in the marketplace. Various definitions of product types are shown below.
Table 1. Various Health and Herbal Product Definitions
Traditional systems of therapy including homeopathy, Natropathy, Ayurveda, Sidha, Unani, Chinese traditional medicine (TCM) and various regional Asian traditional medicine disciplines are rapidly growing in popularity. Until now these complementary medicines have not been regulated in South East Asia, but the government regulation is now rapidly being put in place to set down minimum qualifications for practitioners under registration systems.
Herbal Medicines are very popular in the region with a number of local herbs being aggressively marketed and accepted by consumers. For example in Malaysia, tongkat ali (Eurycome longfolia Jack) is currently one of the most popular herbal medicines and is processed into teas, medicines and even coffee. The product is marketed over the country by numerous local companies. According to ethnobotany literature, the herb is believed to be an energy booster, aphrodisiac and anti malaria remedy. kacip fatima (Labisia pumila) is another herb used to treat dysentery, rheumatism and women’s ailments at birth. Mengkudu or noni (Morinda citrifolia) is used to lower high blood pressure and lessen cancer risks.
Table 2. Examples of Nutraceuticals and Herbal Medicines
As there are still a vast number of plants and trees yet to be examined for potential in phyto-medicine, there is currently great interest from government research institutions, private companies and consumers in this area. There are many research paths that can be pursued in the herbal area. However, basic to herbal development is bio-prospecting for potential new plants that can be developed into traditional medicines, pharmaceuticals, phyto-pharmaceuticals, nutraceuticals or flavour & fragrance ingredients. Bio-prospecting is a long term program, where results will only come after systematic prospecting. New screening methods available can cut the time through speedy preliminary assays. Bio-prospecting for potential pharmaceuticals, phyto-pharmaceuticals or traditional medicines is primarily concerned with plants that have efficacy in the following major areas; anti inflammatory, anti microbial, anti-cancer, and anti HIV. Other potential efficacy assays may also be selected for potential cosmetic applications like skin whitening, UV absorbing, or anti aging properties etc, depending on the resources available to the research team. Ethno-botanical monographs provide a guide to the team in selecting what other screening assays should be utilised for selected plants, for example anti-malarial or anti-dysentery.
Bio-prospecting is used to find compounds for ‘molecular templating’ as was discussed in chapter one. Many companies find it much easier to synthesise a compound rather than cultivate and develop a new crop. For example, a group led by Jay Keasling at the University of California, Berkley, developed the compound artemisinin from the export of artemisimic acid from GM yeast cells, grown in bio-reactors [160]. This decreases the potential to cultivate Artemisia annua as a source of artemisinin.
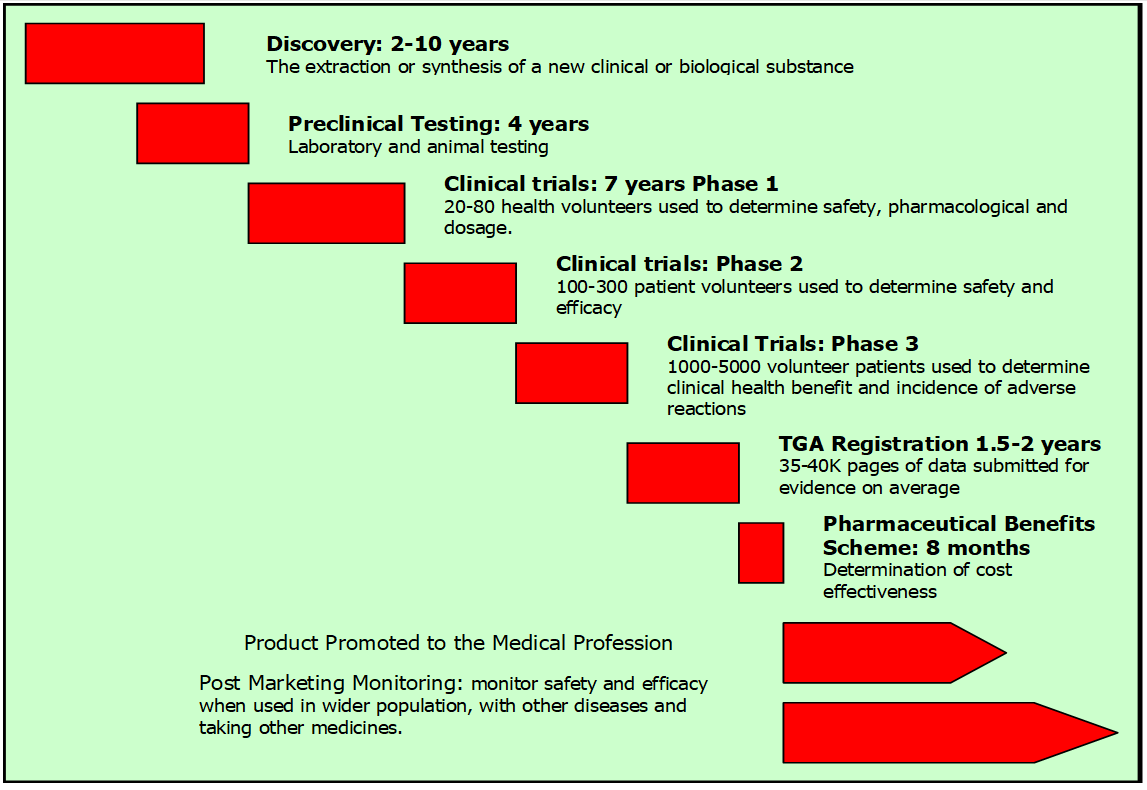
The product development framework for a pharmaceutical product is shown in Figure 1.
Figure 1. The Stages Involved in the Development of a Pharmaceutical Product
The development process of a herbal medicine is simpler than for a pharmaceutical, due to less stringent regulation in most countries. The development of a plant extract as a pharmaceutical product will have to go under the same stringent regime as any other pharmaceutical product, taking many years and costing hundreds of million US dollars to complete, as the above figure indicates.
An exert from Essential Oils: Art, Science, Industry, & Entrepreneurship - A focus on the Asia-Pacific region
Click on subscribe so articles can be directly emailed to your inbox: